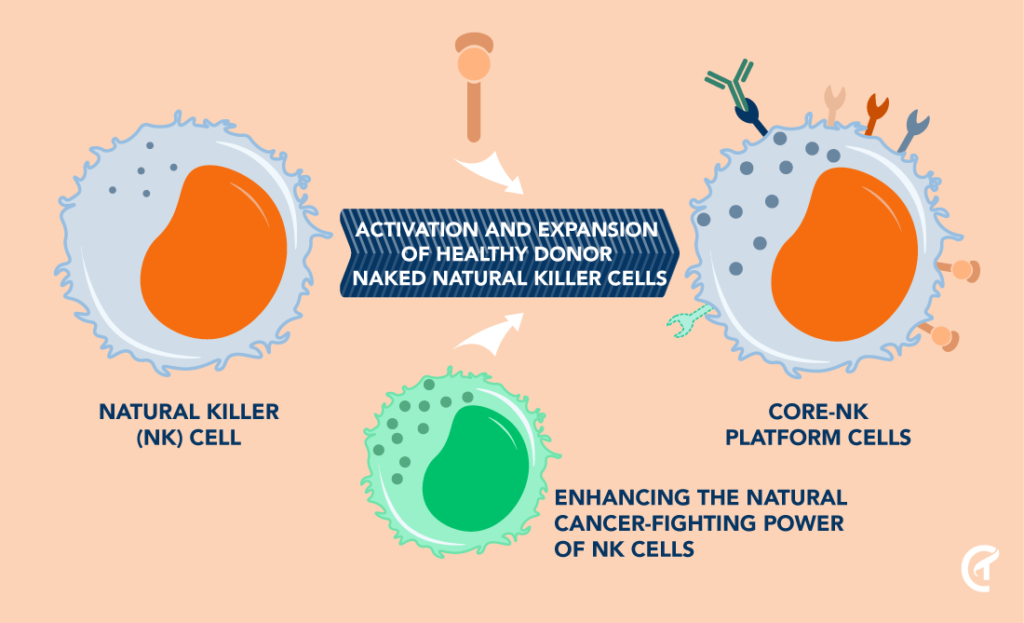
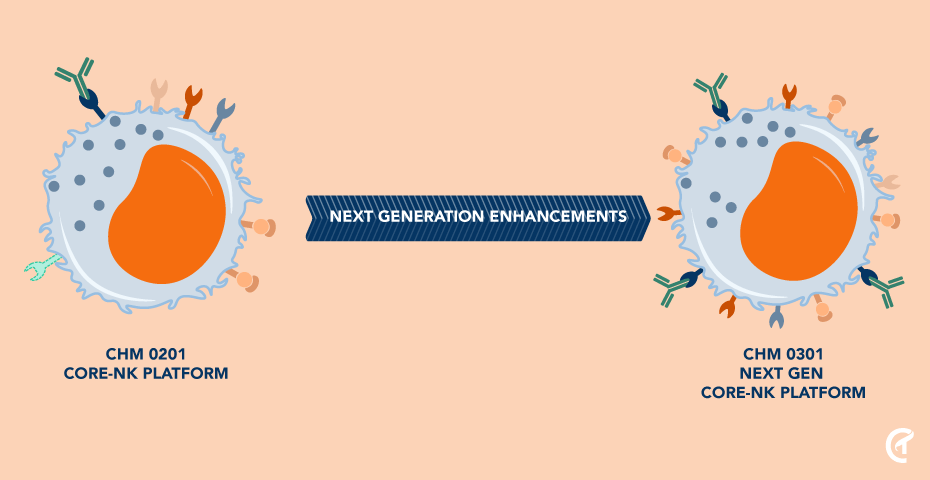
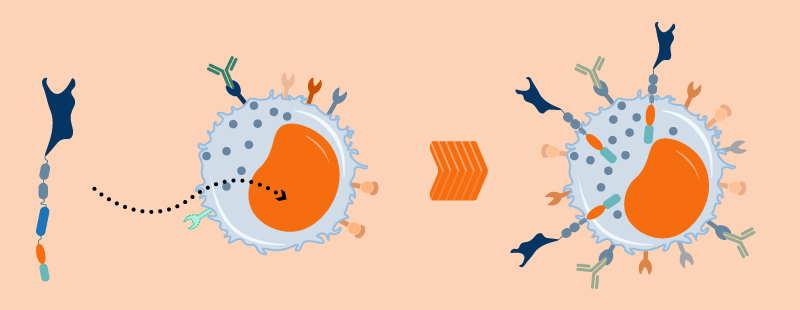
Natural Detection and Direct Killing of Cancer Cells
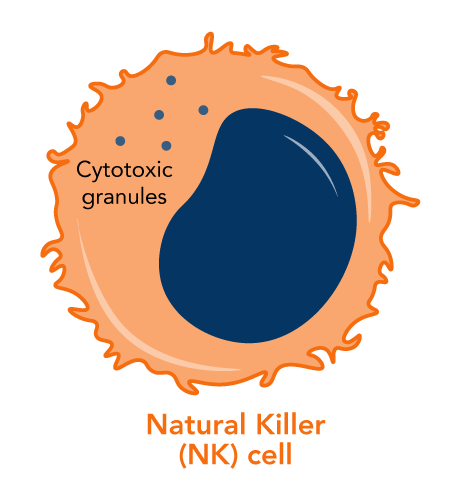
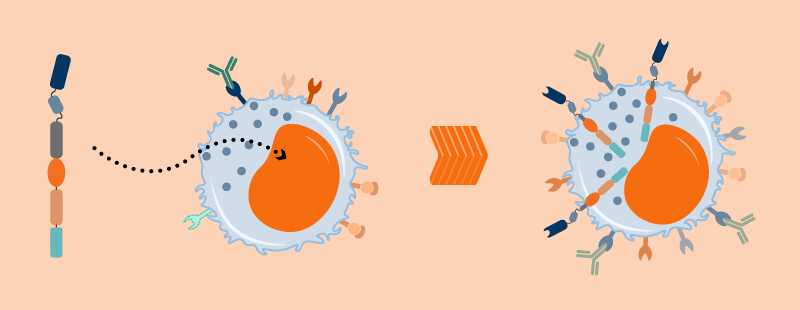
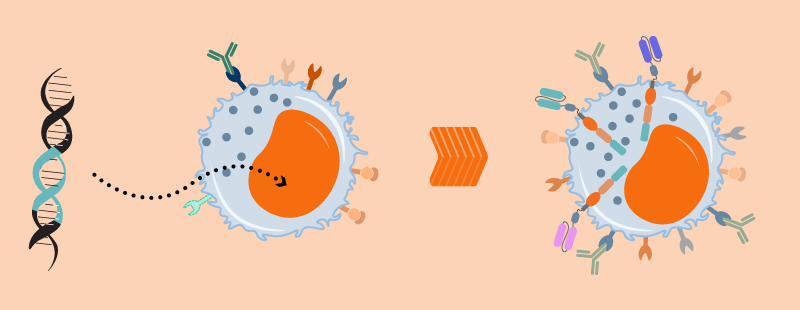
Natural killer cells patrol our bodies looking for abnormal cells. They have an innate ability to recognize and and kill cancer cells without any priming or prior activation. (They’re named for this “natural” killing ability).